What are mitochondria?
Mitochondria perform many functions necessary for cell metabolism, the most important being energy, that is, adenosine triphosphate (ATP) production. This aerobic process unique to mitochondria is called oxidative phosphorylation (OXPHOS), occurring in the respiratory (or electron transport) chain within the membrane’s wall, and utilises carbohydrate, fat, and oxygen.
Approximately 90% of the energy required for life, is via the various 70+ polypeptides or proteins interacting within this respiratory chain (RC). Most nucleated cells contain 500 to 2,000 mitochondria each, though some contain only several, with each being distinct in size, shape, and metabolic function for the specialised cell type harbouring it. Mature red blood cells do not contain any mitochondria, though its precursor, the proerythroblast, does and is critically dependent upon them.
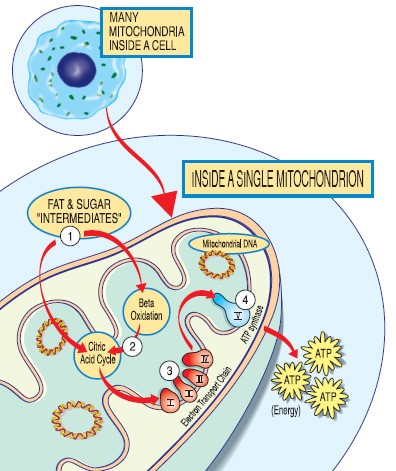
(Diagram – sourced from MDA and The Mitochondria Research Society)
What is it?
Mitochondrial diseases (primary) are a clinically heterogeneous group of disorders that result from a genetic variant (>350 identified) directly causing RC dysfunction, and is the most common group of inherited metabolic disorders. The dysfunction may also be secondary to other intracellular metabolic defects, but are not included in this definition.
Consequently, tissues and organs highly dependent upon aerobic metabolism are most commonly affected in mitochondrial disease, and include the muscles, brain, heart, liver, gastrointestinal tract, ears, and eyes. Organ dysfunction occurs when its energy-production cannot meet its energy demands, with symptoms occurring or becoming exacerbated by the smallest decreases in energy production, or small increases in energy demands.
Mitochondrial disorders are progressive, and whilst they may only affect a single organ, most involve multiple organ systems, with prominent neurologic and myopathic features. Although they involve ‘any organ, any symptom, at any age’, the earlier the presentation, the poorer the prognosis is generally, with no consistent correlation between the severity of the biochemical dysfunction and the patient’s clinical presentation.
Classification may be either:
- phenotypically with affected patients displaying a cluster of distinct clinical features (e.g. MELAS, MERRF, LHON),
- genotypically, as per the underlying genetic variant (e.g. POL-G),
- biochemically, dependent upon the resultant RC dysfunction (e.g. CoQ10 deficiency)
- historically, named after the discovering clinician (e.g. Pearson, Leigh), or
- void of fitting into one particular category (e.g. general mitochondrial myopathies)
Mode of inheritance?
Mitochondria are unique in that they have their own DNA called mitochondrial DNA (mtDNA), distinct from nuclear DNA (nDNA). The mitochondrion is encoded by approximately 1,500 genes in total, with mtDNA comprising just 37 of those genes, and the rest being nuclear. Less than 10% of those 1,500 genes are allocated to ATP production, and with variants occurring in either mtDNA or nDNA, potentially complicated by interactions between the two groups, genetic analysis of mitochondrial diseases often becomes complex.
Variations in nDNA are inherited by ‘autosomal inheritance’, usually in a ‘recessive’ Mendelian pattern from either parent or both. Approximately 75% of primary paediatric mitochondrial disease is autosomal recessive, presenting early and commonly fatal. Less commonly the autosomal inheritance may be ‘dominant’ with a genetic variant from either parent producing illness.
Variants in the mtDNA are maternally inherited, due to the sperm’s mtDNA, generally being omitted or destroyed during conception, and most commonly account for adult mitochondrial disorders. ‘Homoplasmy’, is when all mtDNA copies are identical within a cell, whereas ‘heteroplasmy’ is when a mixture of variant (dysfunctional) and wild-type (normal) mtDNA exists, as demonstrated in those with mitochondrial disorders. The proportion of mtDNA variant differs amongst patients, (including those within the same family), and even within the organs and tissues of each person affected. When the mtDNA variant exceeds a critical proportion, ‘the threshold effect’, the biochemical abnormality of the mitochondrial RC is therefore expressed. This biochemical abnormality and its subsequent phenotype (or clinical expression), varies according to:
- each individual’s genetic background,
- each variant’s intrinsic pathogenicity,
- each variant’s distribution amongst the differing organs and tissues,
- the ‘point-in-time’ aerobic energy-demand of those differing tissues or organs, and
- epigenetics and the nDNA-mtDNA interplay, whose increasingly significant roles are emerging through continuing research.
Maternal Inheritance of mtDNA variants
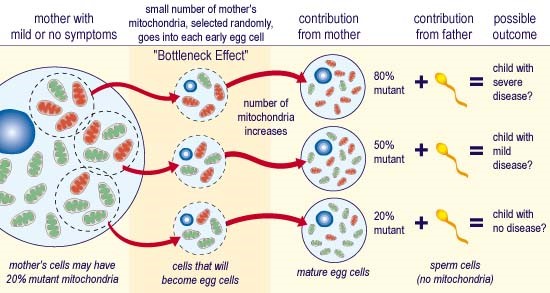
Incidence
Conservatively, a mtDNA variant is present in 1 in 200 of the general population, which equates to 10+ patients for each full-time general practice of 2000+ patients. Whilst many may present with no to minimal symptoms, many others with mild to moderate symptoms, are left confounded as to the reason for their bad luck or bad health, or that presenting within other family members, including their own children.
Severe illness presents in at least 1 in 5000 of the general population and is more common amongst the childhood presentations. This is particularly relevant in the USA, when every 30minutes a child is born, who by the age of 10y will develop mitochondrial disease.
What are the symptoms?
A suspicion of a mitochondrial disorder results from good clinical acumen, an analysis of the spectrum, clustering, and quality of presenting symptoms, with particular attention directed towards any ‘atypical‘ or inadequately applied alternative diagnoses. Please see below for the comprehensive list of mitochondrial symptoms, tabulated into the key systemic presentations.
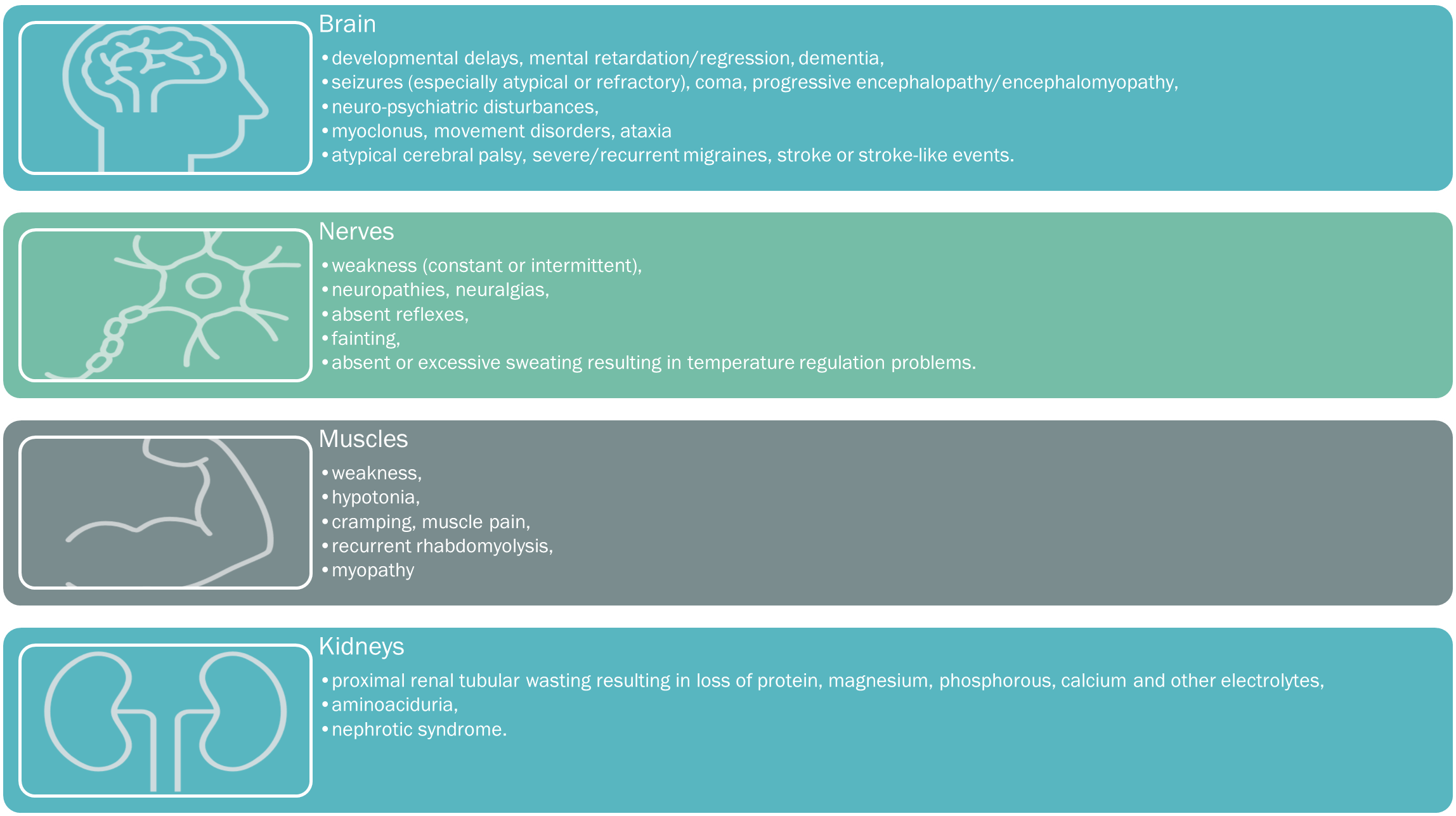
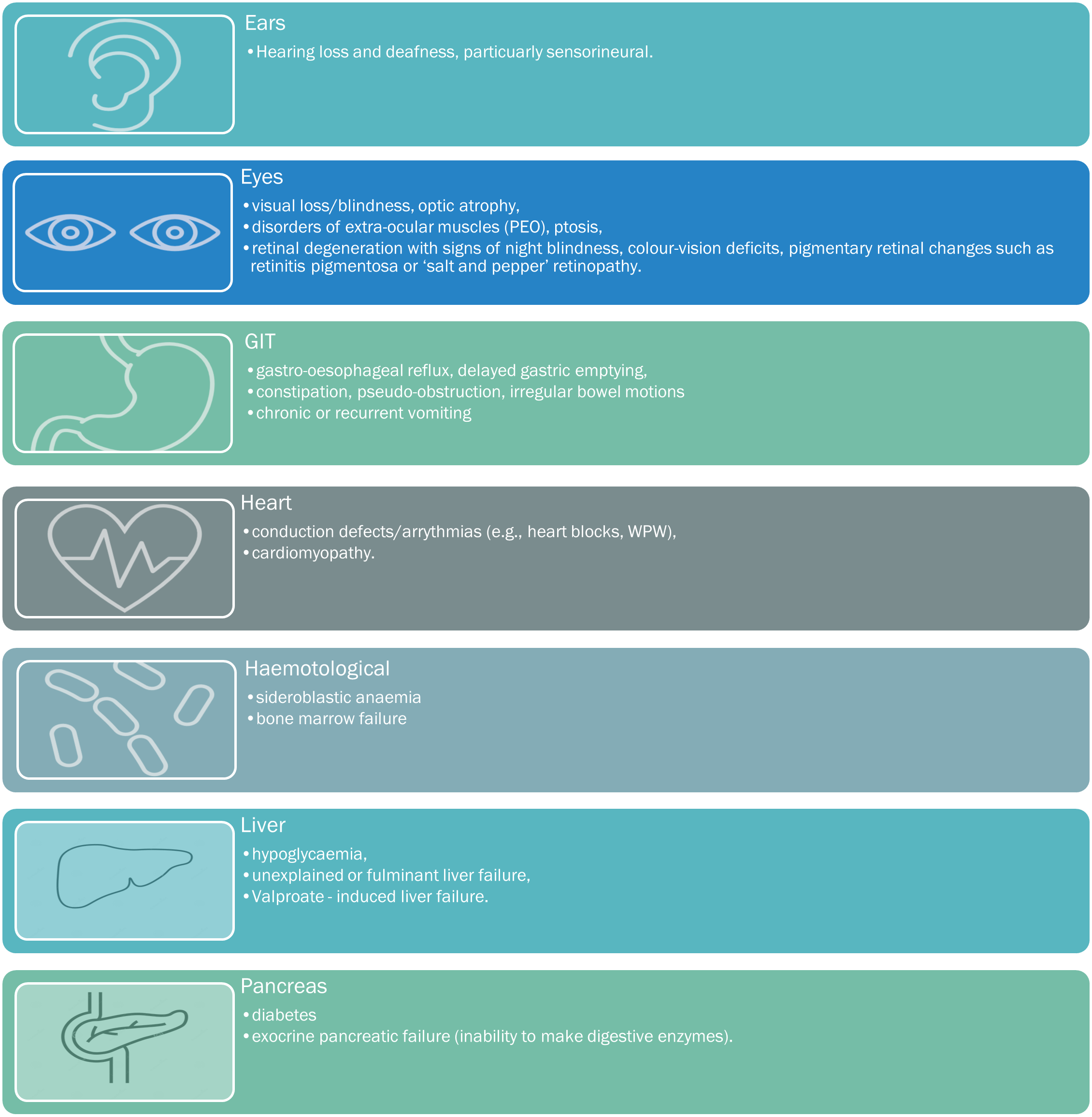

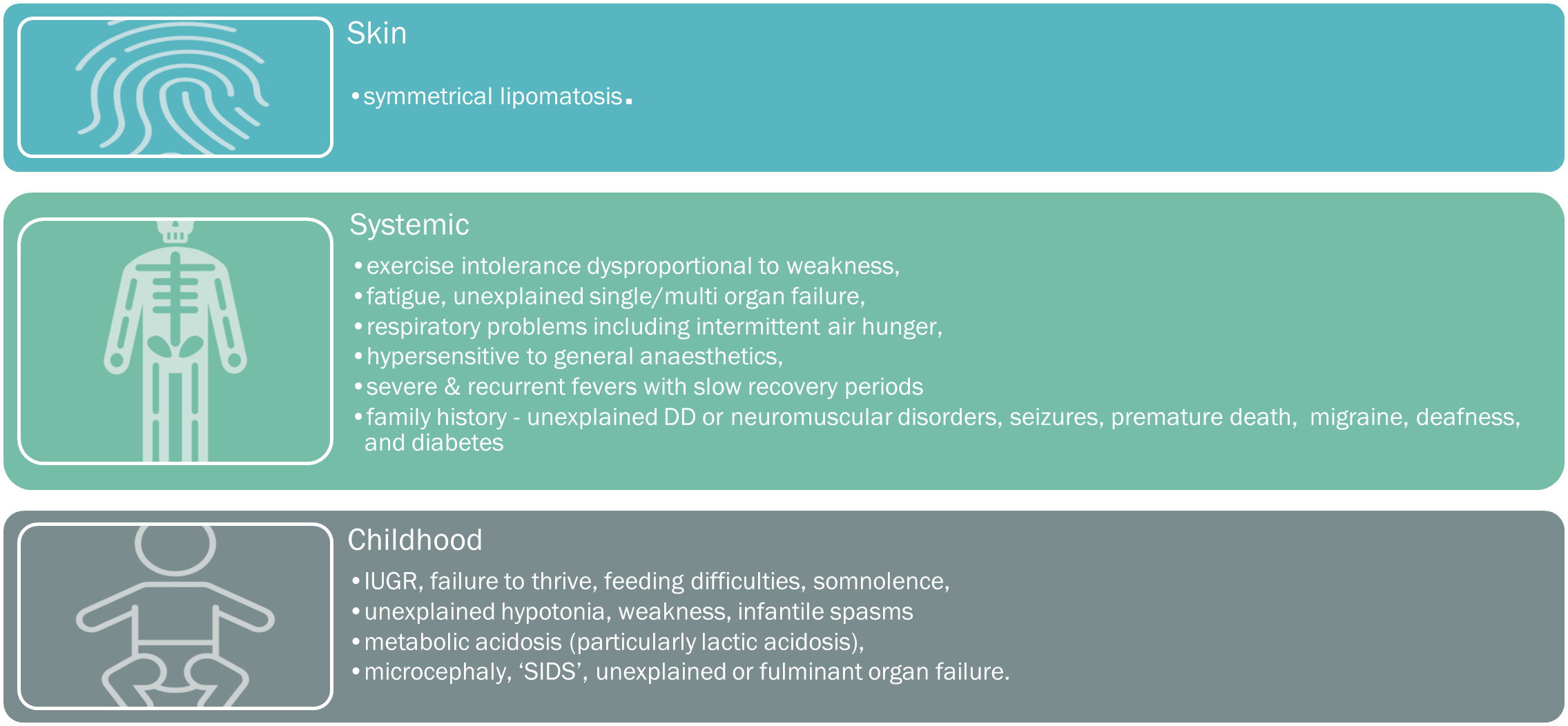
When to suspect mito
The increasing number of phenotypes and genotypes discovered, variability in ‘same variant’ presentations, complexities of the genetics, and the diagnostic odyssey, often presents many challenges to the physician.
However, an awareness of the mitochondrial RED FLAGS (below), can help alert an astute clinician to a potential diagnosis.
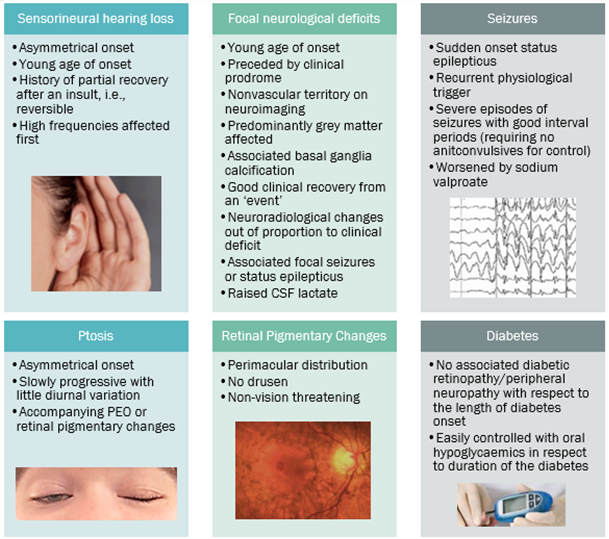
Common Phenotypes
Some of the more common PHENOTYPES include:
PEO (Progressive External Ophthalmoplegia)
MELAS (Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like episodes)
Leigh syndrome (Subacute necrotizing encephalomyopathy)
LHON (Leber Hereditary Optic Neuropathy)
KSS (Kearns-Sayre Syndrome)
MERRF (Myoclonic Epilepsy with Ragged-Red Fibres)
MNGIE (Mitochondrial Neuro-Gastrointestinal Encephalopathy)
POL-G variant – causing numerous related disorders
Diagnostic Pathway
STEP ONE – the possibility of mitochondrial disease is considered when the caring family physician recognises;
• any RED FLAGS of mitochondrial disease,
• a distinct cluster of symptoms, especially neuromuscular, ocular (ptosis and PEO), and sensorineural hearing loss,
• a family history with similar or overlapping features (often left undiagnosed),
• a ‘common’ condition that has ‘atypical’ features, setting it apart,
• 3 or more organ systems involved that are energy-dependent in character and of a quality distinct to mitochondrial disease (atypical, unusual, non-descript, and/or unexplained), or
• a chronic condition with clinical fluctuations, recurrent setbacks, and exacerbations, frequently triggered by physical stressors such as surgery and/or infections.
STEP TWO – before any referral to a specialist, a clinical suspicion of mitochondrial disease is made complete by the GP performing a:
• comprehensive review of all symptomatology,
• family history of all diagnosed or undiagnosed ‘family traits’, and
• ‘full systems’ review to capture any potentially relevant unidentified features.
Clinical investigations organised by the GP, then detects, clarifies, and confirms any symptomatology elicited during the history taking and physical examination. For example, constipation (slow bowels) is often not noted by the patient, but instead by bowel transit studies.
Dependent on the accessibility of the GP and patient to specialised services, some investigations may best be left for the neurologist, but should include:
• Full Biochemical workup/FBC
• Glucose tolerance test with insulin levels
• Formalised hearing assessment
• Ophthalmological examination
• ECG/24h-holter/echo
• Bowel transit studies/gastric emptying studies
• Electrophysiology studies, particularly EEG, (also NCS, EMG, CT, MRI/MRS, CSF analysis, etc – most often performed by reviewing neurologist)
• Allied health team assessments (occupational therapist, speech pathologist, physiotherapist), and
• ‘ERRORS OF METABOLISM” INDICATORS‘ – best organised by specialist and include: emerging biomarkers, blood lactate, serum alanine, urinary Krebs cycle intermediates, lactate/pyruvate ratio, hyperammonaemia, urinary organic acids.
STEP THREE – referral is then made to a neurologist, neurogeneticist, paediatric neurologist, paediatrician, clinical/metabolic geneticist (often via a paediatric review first), or metabolic physician, with all clinical and investigative suspicions referred to in the attending letter.
Currently, mitochondrial disease genetic testing is not a MBS reimbursed item, except in children <10years with:
A) moderate global developmental delay or intellectual disability, or
B) dysmorphic facial appearance and 1 or more major structural congenital anomalies.
For all other children or adults, a specific ‘candidate gene test’ may be performed by an “expert mitochondrial specialist”, but only where the clinical presentation conforms to a recognised phenotype/syndrome and is dependent upon the remunerating hospital’s approval or funded by the patient or child’s family. If negative or non-confirmatory, ‘whole genome sequencing’ (WGS) would then be required to identify the pathogenic nDNA or mtDNA variant, but is often not pursued due to funding difficulties.
Otherwise, mitochondrial disease is diagnosed on ‘clinical suspicion only’, supported by evidence from symptomatology, physical examination, investigations, imaging, and tissue/RC analysis (most commonly via muscle biopsy, but also skin, bowel, or liver).
STEP FOUR – when a ‘confirmed or highly suspicious’ diagnosis has not been obtained, patients should continue regular reviews, with ongoing assessment for further clinical deterioration and new organ involvement, as even the most classical mitochondrial syndromes may require time to become evident.
To further both aid, identify and unify a mitochondrial disease diagnosis, various internationally accepted diagnostic criteria standards have been developed and such examples include the Nijmegen, Bernier, or Walker criteria.
Management Principles
• the dignity of a diagnosis (it is not imaginary),
• access to services/resources (NDIS, CentreLink),
• better quality of life from applying appropriate management principles,
• appropriate understanding and monitoring of the illness,
• prevention or minimisation of metabolic crises,
and where a genetic variant can be identified:
• earlier more appropriate and therefore effective management can be commenced, to halt or delay disease progression, prevent, and minimise metabolic crises, with the overall outcome of a better quality of life,
• targeted treatments and key strategies can be applied to optimise symptom management,
• inappropriate, ineffective, and potentially harmful treatments can be ceased,
• targeted clinical monitoring allows earlier identification and prevention of complications,
• informed and accurate family planning avoids the inheritance of variants,
• alternative ‘artificial reproductive techniques’ can be offered, and
• family members can be informed of clinical and inheritance risk factors,
The GP has an effective and vital role in the patient’s care which can be further enhanced by learning about the illness, asking questions of the team overseeing the management, and by supporting and advocating for the patient when they are engaging with other medical providers/services less familiar with the illness.
Living with mito
The often delayed and protracted disease progression amongst adults, carries with it an increasing burden of disease, and its own significant and subsequent impacts on the patient and their carers, such as:
- loss of self-image, self-love, identity, and independence,
- altered relationships and social interactions,
- diminished quality of life and physical discomfort,
- diminished work, financial stresses, and increased expenditures,
- grief (in regard to all losses and future uncertainties), and
- the emotional accommodations required to continue.
Although there is no cure and ‘little to no’ capacity to improve RC function, management principles are instead directed at optimising current energy production capabilities and reducing energy losses, whilst meeting lifestyle needs, preventing metabolic crises, minimising harm, slowing disease progression, and monitoring for complications.
Lifestyle management principles include:
• the avoidance of cellular stresses where possible, such as illness, infections, fatigue, poor nutrition, fasting, excessive temperature changes, and surgery,
• best practice management of cellular stresses when avoidance is not possible, to minimize metabolic crises, such as applying anaesthetic guidelines to mitochondrial patients requiring surgery,
• earlier and appropriate metabolic crises management, through utilizing emergency letters, alert bracelets, and health summaries with the contact details of overseeing team and caring specialists,
• the alleviation of symptoms through medical, surgical and supportive management,
• adequate rest and sleep, pacing yourself through your daily activities,
• adequate nutrition, with a balanced diet eaten as 5-6 small meals daily,
• the avoidance of mitochondrial toxins and contraindicated medications,
• allied health support through such services as physio, speech therapy, exercise physiologist, psychologists, OT, and,
• the addition of supplements such as the ‘mitochondrial cocktail’ (e.g., CoQ10, Mg Orotate, alpha lipoic acid, Vit E, B’s & C), and others such as Idebenone, NAD supplements, nicotinamide riboside, L-carnitine, creatine, and L-arginine.
Prognosis
Future Research
New and improved diagnostics are also key to the future direction of mitochondrial disease as it is estimated that >90% of patients remain undiagnosed. Despite the emergence of new biomarkers such as FGF21 and GDF15, the quest for funded WGS testing becoming more urgent, the discovery of numerous new pathogenic variants annually, the availability of more accurate and sensitive imaging techniques, and more advanced clinical diagnostic criteria, mitochondrial diagnostics is progressing but still has much to achieve to equal the detection rates of other mainstream illnesses.
Mitochondrial Donation
Mitochondrial donation is an IVF-based assisted reproductive technology, that has the potential to prevent mtDNA disease in the offspring of mothers harbouring the variant. Research in this area is not entirely new, and the UK altered their legislation to allow the technique to be used in mothers meeting certain and strict criteria.
There are four mitochondrial donation techniques that allow an embryo to be created from the nDNA of the biological parents, whilst utilising the mitochondria (containing the ‘healthy mtDNA’) from an egg donated by another woman, that is, the ‘mitochondrial donor’. In April 2022, Australian government processes allowed for a change in legislation which has now legalised mitochondrial donation, and so be offered to potential mothers possessing a mtDNA pathogenic variant, in the format of a ’10 year clinical trial’. For more up to date information, see the Australian Government website for details, at: https://www.health.gov.au/initiatives-and-programs/mitochondrial-donation#about-mitochondrial-donation
Further Information
As the Australian Mitochondrial Disease Medical Network endeavours to develop its resource base, other well-stablished, clinically orientated and trusted organisations are available for further information and include:
- UMDF (United Mitochondrial Disease Foundation) in the USA: https://www.umdf.org/
- Rare Mitochondrial Disorders Services in the UK: https://mitochondrialdisease.nhs.uk/
- Mitochondrial Medical Society in the USA: http://www.mitosoc.org/
- The Wellcome Trust Centre in the UK (associated with Newcastle University): https://www.newcastle-mitochondria.com/
Also listed are more consumer-orientated but also well-established, trusted and informative groups including:
- Mito Foundation in Australia: https://www.mito.org.au/
- International Mito Patients based in The Netherlands: https://www.mitopatients.org/
- Mitoaction in the USA: https://www.mitoaction.org/
- the Lily Foundation in the UK: https://www.thelilyfoundation.org.uk/
- Mitocanada in Canada: https://mitocanada.org/
